Clinical Laboratory Improvement Amendments
Clinical Laboratory Improvement Amendments of 1988 amended the Public Health Service Act to extend jurisdiction of the Department of Health and Human Services to regulate all laboratories that test human specimens for the purpose of providing information for diagnosis, prevention, or treatment of any disease or impairment of, or the assessment of the health of, human beings. All information can be found in CMS Internet Only Manual , Publication 100-08, Chapter 15, Section 15.4.2.2
Does Cpt 87804 Require A Qw Modifier
4/5modifier QWCPTrequiringQW
Also question is, is CPT code 87804 CLIA waived?
Remember that 87804 is a Clinical Laboratory Improvement Amendments waived test, meaning you need to have CLIA certification to perform these flu tests. Further, you must append modifier QW CLIA–waived test to each 87804 entry on your claim to indicate your CLIA status.
Beside above, can CPT 87804 be billed twice? Use 87804 coding rule while testing for strains A & BIf you use a product that distinguishes between influenza A & B and the doctor documents both results, you should code 87804 twice. Technically it’s two tests just done in one so you’re right in billing twice since the physician is documenting two results.
Then, does CPT code 87400 need a modifier?
payers differ on modifiers, if they do not accept modifier -59, append modifier -91 . Code 87400 is taken fron any other source, by culture or any technique not considered rapid flu test.
Does CPT 87880 need a modifier?
You don’t want to use the code for strep test done by culture without the QW modifier when you have performed the quick strep test done by kit , which is on the CLIA-waived list.
My Laboratory Performs Urine Drug Screening For Employment Purposes Is Clia Required
CLIA is not required for laboratories performing drug testing for employment purposes only. The Louisiana regulations for this type of testing can be found in L.A. R.S. 49:1001-1021. The Louisiana Department of Health does not regulate Urine Drug Screening Laboratories. CLIA only applies if the patients are being sent for treatment as a result of such testing.
Recommended Reading: Does Medicare Pay For Ambulance Calls
Does Louisiana Require A Physician Order To Perform Laboratory Testing
No. CLIA regulations allow patients to have tests performed by certified laboratories without a physician order. This is known as Direct Access Testing . There are no state laws in Louisiana that prohibit this. However, there are certain restrictions in Medicare and in some insurance coverage that disallows tests not ordered by physicians. Therefore, some individual laboratories within the state may have their own restrictive guidelines relative to ordering tests.
How To Apply For A Clia Certificate Including International Laboratories
APPLICATION FOR A CLIA CERTIFICATE: FORM CMS-116
The Centers for Medicare & Medicaid Services has made available the Clinical Laboratory Improvement Amendments of 1988 Application for Certification Form, CMS-116. This form should be completed and mailed to the address of the localState Agency for the state in which your laboratory resides. Refer to theState Agency & Regional Office CLIA Contacts pagelocated on the left navigation bar to obtain the appropriate state agency address. The CMS-116 form and its instructions are found as a download in the Related Link Inside CMS section below and contain the following information:
- The CLIA application form, CMS-116
- Instructions for completing the CMS-116
- Guidelines for counting tests for CLIA and
- Tests commonly performed and their corresponding laboratory specialties/subspecialties.
NOTE FOR INTERNATIONAL LABORATORIES: The CLIA certification process for international laboratories is outlined in the download below . If your laboratory is outside of the United States, or is not one of the territories of the United States and is seeking CLIA certification, before completing a CMS-116, you should contact
The CLIA application collects information about a laboratory’s operation, which is necessary to determine the type of certificate to be issued and the fees to be assessed.
NOTE FOR WASHINGTON STATE APPLICANTS: You should not complete the CMS-116 form but should contact the Washington State Agency at 395-6746 for guidance.
You May Like: Does Medicare Pay For Blood Pressure Cuffs
Cpt Code 82947 85610 Guide
procedure Code Description85610 Prothrombin timeNOTE:DescriptionPROTHROMBIN TIME ICD-10 Description ICD-10 ICD-9What is the payment amount for PT/INR testing in those instances in which Medicare coverage in available?CLIA: Laboratory Tests related denials – CO-B7CO-B7:82947 and 85610Resolution85610 Prothombintime85610QW April 15, 2010 CoaguSense Self-Test Prothrombin Time/INR Monitoring System Note:Modifier QWDescription CLIA-waived testCPT CODE TEST NAME MANUFACTURER USE 85018QW
Reminder For Blue Medicare Providers To Submit Your Clia Certificate Numbers
Blue Cross and Blue Shield of North Carolina requests that providers participating in our Medicare Advantage networks submit the Clinical Laboratory Improvement Amendments certificate number for your practice, hospital-based laboratory, or reference laboratory. A copy of your CLIA certificate is also acceptable.
This request applies to all CLIA-certified providers, including hospital-based laboratories and reference laboratories, within our system. If you have previously responded to this request on or after March 1, 2016, please do not send in a duplicate copy.
Lab services claims should be submitted in accordance with CMS guidelines and only for services covered under the billing NPI CLIA certificate. Please include the CLIA number on all claim forms.
To submit a copy of your CLIA certificate or your certificate number:
Read Also: What Age Do You Register For Medicare
Who Can Qualify As The Laboratory Director For A Certificate Of Provider Performed Microscopy Procedures
Only a physician , a dentist, and a midlevel practitioner qualify as a laboratory director for a Certificate of PPM. Only these individuals can perform PPM procedures under this certificate. Other individuals can only perform this testing under a Certificate of Compliance or a Certificate of Accreditation. See the Center for Disease Control resources for more information PPMP.
Do I Need To Obtain A Clia Certificate
Generally, CLIA mandates that all the facilities performing even one of the applicable tests on any type of materials derived from the human body such as blood, body tissue, etc. for diagnosis, prevention or treatment of any disease, or for the assessment of health, need to conform to certain federal requirements.
If a facility/organization performs any such applicable test, it will be considered as a laboratory test as per CLIA and the facility will generally have to obtain a CLIA certificate that corresponds to the complexity of these tests. In addition to this, there is a point to be noted that you will not require to obtain a CLIA certificate if you are located in any of the states of Washington or New York, as these states have their own operated laboratory regulatory programs. You are required to contact the appropriate agency in the state to determine whether you require a CLIA certification or not.
Don’t Miss: How Do I Cancel Medicare Part A
Who Needs A Certificate
According to the CLIA regulations, any laboratory that conducts even a single test on materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or the impairment of, or assessment of, the health of human beings is required to obtain a CLIA certificate. The objective of the CLIA program is to ensure quality laboratory testing.
What Is A Clia Waiver
A Certificate of Waiver is one of four types of certificates issued under CLIA, and is the type to request if you plan to conduct only waived rapid HIV tests. Sale is restricted to clinical laboratories
Also to know is, what does it mean to be CLIA waived?
To be waived means that certain tests can be performed without the need for the conduct of more stringent standards imposed by CLIA. The FDA classifies as waived those tests that employ relatively simple methodologies such that when performed properly, these tests are least likely to yield erroneous results.
what are examples of CLIA waived tests? Examples of POCT include, but are not limited to:
- Glucometers for measuring blood sugar.
- Handheld chemistry analyzers to perform tests such as creatinine, electrolytes, hemoglobin, troponin, cardiac enzymes, and blood gases.
- Dipsticks for urine chemistry testing.
- Testing strips for vaginal pH.
Herein, do I need a CLIA waiver?
Yes, the testing you perform qualifies as waived laboratory testing, and you need a CLIA Certificate of Waiver. This testing requires a CLIA certificate regardless of how many tests you perform and even if you do not charge the patient or bill Medicare or other insurances.
What is CLIA and what is its purpose?
You May Like Also
Recommended Reading: Are Lidocaine Patches Covered By Medicare
What Is Clia Number
Jun 21, 2010 | Medical billing basics
Clinical Laboratory Improvement Amendments CLIA Quality Standards CLIA Number When is a CLIA Certificate Required?DO I NEED TO HAVE A CLIA CERTIFICATE?WHAT IS A WAIVED TEST?HOW CAN I FIND A LIST OF WAIVED TESTS?HOW DO I APPLY FOR A CLIA CERTIFICATE?ARE THERE ANY STATES THAT EXEMPT ME FROM HAVING TO APPLY FOR A CLIA CERTIFICATE?IF I HAVE MORE THAN ONE LABORATORY LOCATION, DO I NEED A CLIA CERTIFICATE FOR EACH LOCATION?WHEN CAN I BEGIN TESTING?WHERE CAN I FIND ADDITIONAL INFORMATION AND GUIDANCE?Why Is CLIA Important? Does CLIA Apply Only to Laboratories Obtaining Payment Through Medicare?What Is a Certificate of Waiver ?CLIA CertificationWhen is a CLIA Certificate Required?DO I NEED TO HAVE A CLIA CERTIFICATE?WHAT ARE THE DIFFERENT TYPES OF CLIA CERTIFICATES AND HOW LONG ARE THEY EFFECTIVE?
What Is A Clia Id
4.8/5CLIACLIA
Keeping this in consideration, what is CLIA ID number?
A laboratory that is included in the CLIA legislation must obtain a CLIA certificate from the U.S. Department of Health and Human Services. The certificate will include a 10-digit number, which is your CLIA number.
Similarly, who needs a CLIA certificate? CLIA requires that any facility examining human specimens for diagnosis, prevention, treatment of a disease or for assessment of health must register with the federal Centers for Medicare & Medicaid Services and obtain CLIA certification.
Also to know, what is CLIA and what is its purpose?
In general terms, the CLIA regulations establish quality standards for laboratory testing performed on specimens from humans, such as blood, body fluid and tissue, for the purpose of diagnosis, prevention, or treatment of disease, or assessment of health.
What are the 3 levels of CLIA testing?
The basis of the complexity of CLIA tests are categorized into three levels: waived tests, moderate and high complexity.
You May Like: When Can I Start Collecting Medicare Benefits
What Is Required To Become A Clia Certified Lab
Normally, a lab fills out a government form CMS-116 and joins the CLIA program through their state office. This is important for physician offices who conduct routine tests such as glucose blood tests or urine strips. Despite the in-person and office-based nature of these tests, even small-scale testing such as these counts as lab testing. These are called waived tests. The rules are different in New York and Washington State since these states run their own lab testing programs. Contact your local CLIA state office for details. More details on CLIA waived tests and CLIA laboratory programs can be foundhere.
How Can I Renew My Clia Certificate If I Have Not Received A Renewal Notice Form Cms
If you have lost or misplaced your fee coupon, you can still submit your payment without it. To submit a payment you should make a check payable to CLIA LABORATORY PROGRAM in the amount that is owed for your specific certificate type. Mail the check to:
PORTLAND, OR 97208-3056
Make sure you write the CLIA number clearly on the front of the check to ensure it gets credited to your account.
CMS now has the capability of accepting electronic payment or credit card transactions.
You May Like: Is My Spouse Covered Under My Medicare
Certificate Of Clia Waiver Basics
A CLIA Waiver Certificate is legal proof that a particular testing laboratory performs only waived tests and is allowed to bypass certain CLIA regulations.
With a Certificate of CLIA Waiver, a lab is qualified to conduct waived laboratory tests. The validity of the certificate is two years.
This means the lab can only conduct simple testing that involves uncomplicated laboratory procedures and examinations. Tests other than waived tests cannot be performed.
Labs with a Certificate of Waiver still must meet the following requirements under CLIA:
- Enroll in the CLIA program
- Pay applicable certification fees biannually
- Follow the manufacturers instructions to conduct tests
To obtain a CLIA Certificate of Waiver, the lab must fill out and submit the CMS-116 Form on the U.S Department of Health and Human Services website or from your local State Agency.
How Clia Differs From Cap
CAP is a type of accreditation, and CLIA is a certification both are responsible for validating high lab testing standards.
The College of American Pathologists is an accreditation organization with the authority to accredit laboratories performing tests on human beings and human specimens.
Under the CAP accreditation program, CAP inspection is performed in laboratories to ensure accurate test results, quality assurance, quality control, timeliness, and accurate patient diagnoses.
CLIA, on the other hand, is a set of guidelines, which are to be followed by any functional laboratory. CAP-accredited labs exceed CLIA requirements and assure the most comprehensive laboratory standards. To obtain CAP accreditation, compliance with CLIA standards is needed.
Don’t Miss: How Much Does Medicare Part B Cost For A Couple
Does Clia Have A Checklist Of Requirements To Assist With Maintaining Compliance
No. There is no checklist available. To assist with compliance issues you should reference the CLIA regulations. Appendix C, Survey Procedures and Interpretive Guidelines for Laboratories and Laboratory Services will also be helpful as it outlines the CLIA survey process. A list of items to be reviewed during a CLIA survey can be found in the Survey Information Packet.
How To Obtain A Clia Waiver
The first step is to know all the right guidelines that are to be followed and what you can expect. Begin by reviewing the official Regulations and Guidance, a document that provides a detailed view of the CLIA Waiver protocols, applications, and categorization procedures.
If you are a device manufacturer, then consider these respective CLIA Waiver application recommendations.
The next step is to check if your lab and respective personnel fulfill the waiver eligibility criteria. Verify your CLIA qualifications for the waiver before submitting your application.
The CDC has also set recommendations for Good Laboratory Practices for Waived Testing Sites.
If your lab is conducting a clinical study, the advantage is that there is room for a Pre-submission. A planned protocol or study design can be shared with the FDA for early feedback and can be used to later support your application for a certificate of waiver.
Follow these guidelines for more information.
Once youve reviewed your qualifications, you can begin the CMS-116 application form. Download Form CMS-116 and review the detailed instructions on completion on Page 6.
You will be asked to fill in the following details:
Also Check: Why Have I Not Received My Medicare Card
Clinical Laboratory Improvement Amendments Number
Nov 1, 2020Administrative
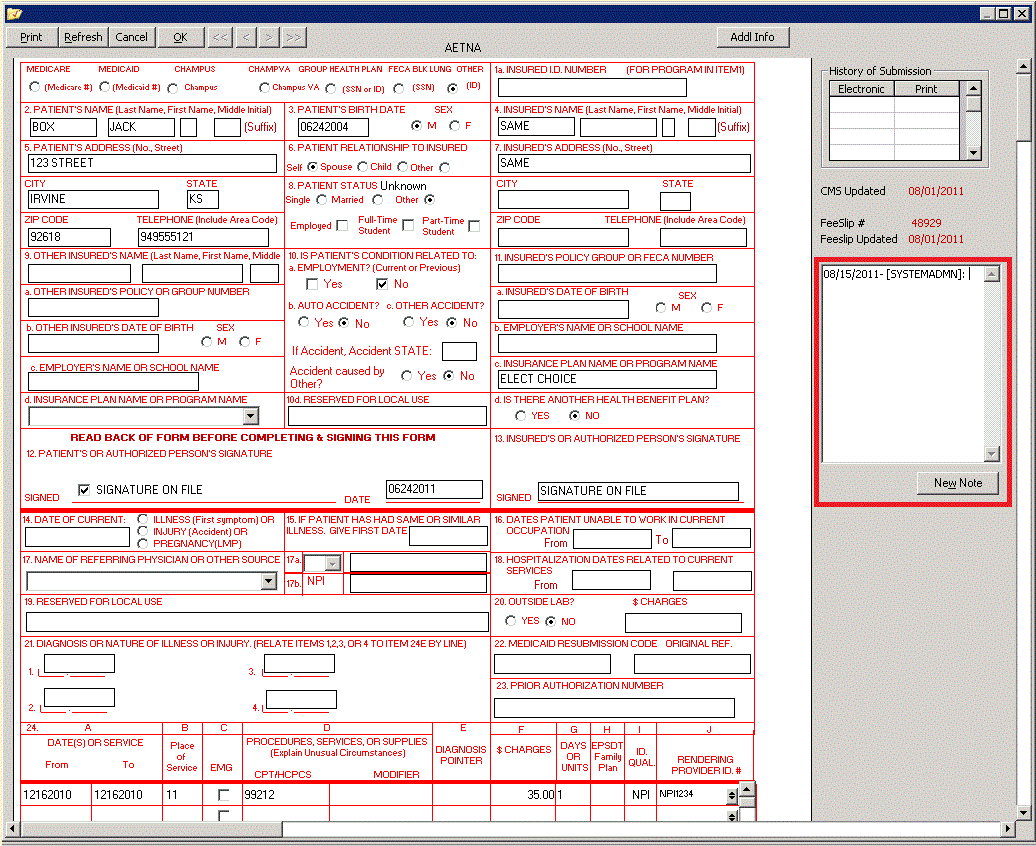
The purpose of this article is to provide additional information regarding submission of the CLIA number on claims for laboratory services that include QW or 90 modifiers. As a reminder, claims filed without the CLIA number are considered incomplete and will reject.
Both paper and electronic claim formats accommodate the CLIA number.
- On the CMS-1500 form, Box 23 is reserved for the CLIA number.
- On the 837P, REF segments are available: REF in loops 2300 and 2400, and REF in loop 2400.
Note: The CLIA number for the Referring Clinical Laboratory should be included in REF
The following examples illustrate how the CLIA number as well as procedure code modifiers QW and 90 should be filed:
|
Claim Format |
Location Reserved for Procedure Modifier and CLIA # |
|
Modifier QW diagnostic lab service is a CLIA waived test |
|
|
CLIA Waived Tests – simple laboratory examinations and procedures that have an insignificant risk of an erroneous result |
|
|
CMS-1500 |
|
|
Loop 2400 SV101-3 |
CLIA #: Loop 2300 or 2400 REF X4 |
|
Modifier 90 Reference Laboratory |
|
|
Referring laboratory refers a specimen to another laboratory for testing Reference laboratory receives a specimen from another laboratory and performs one or more tests on that specimen |
|
|
CMS-1500 |
|
|
Loop 2300 or 2400 REF X4 |
CLIA # – Referring Facility Identification: |
Additional information regarding CLIA is available on the CMS website.
Are There Some Kinds Of Tests That Do Not Require Any Certification

Yes, there are a few exceptions that do not require the certification. Below are mentioned some of the tests for which a CLIA certification is NOT required:
- Blood draws
- Any laboratory that is conducting only Forensic testing
- Drug testing for employment purpose
- Laboratories that are certified by SAMHSA, in which drug testing is performed as per the guidelines issued by SAMHSA. However, a CLIA certificate is required in all the other tests as conducted by a SAMHSA certified laboratory.
In short, if you are not going to perform any on-site testing, you need not obtain a CLIA certificate in such cases. The main purpose of the test will determine whether it requires a CLIA certification or not.
Read Also: Are Hearing Aids Covered By Medicare Part B
How Should I Apply For A Clia Certificate
The CLIA application form is very easily available online . Complete your application and send it to your local state agency in which the laboratory is situated. In addition to this, check for any other specific requirements that might be demanded by your state agencies. If you are not able to find your state agency online then you may contact their helpline number and get the details of your state agency. In case, you are looking forward to any kind of details related to CLIA certification, you should contact your state agency and get the information on priority.
