Mobile Application Software For Self
The effectiveness of mobile phone applications in improving diabetes outcomes has not been established . In a systematic review of 22 trials evaluating mobile phone interventions for self-management , investigators observed a modest decrease in HbA1c levels over a median followup period of 6 months . Studies examining the long-term benefit of mobile phone applications in improving clinical outcomes in diabetes are necessary .
How To Sign Up For Medicare
If you are close to turning 65 and are not getting Social Security or Railroad Retirement Board benefits, you must sign up for Medicare. Even if you are eligible for premium-free Part A coverage, you still must enroll.
If you arent getting Social Security benefits, you will not automatically receive any information from Medicare. You must call Social Security at least three months before the month you turn 65 to avoid any late penalties.
To be eligible for Medicare, you must meet these requirements:
- You are turning 65 or have a qualifying disability.
- You or your spouse worked and paid Medicare taxes for at least 10 years.
- You are a U.S. citizen or permanent legal resident who has lived in the U.S. for at least five years.
- You are receiving Social Security or RRB benefits or have worked long enough to be eligible for those benefits but are not collecting them yet.
You can sign up for Medicare during:
- Your Initial Enrollment Period
- A Special Enrollment Period
- The General Enrollment Period
To enroll, you can:
How Does Medicare Treat Durable Medical Equipment
Medicare treats glucose monitors and blood sugar test strips as durable medical equipment under Medicare Part B. If your physician prescribes glucose monitoring at home, your durable medical equipment costs are generally covered at 80 percent of the Medicare-approved
amount, once your deductible is met.
Keep in mind, however, that under Medicare Part B, your glucose monitoring equipment and supplies will only be covered if both your health care provider and DME vendor accept Medicare assignment. If you live in area affected by Medicares Competitive Bidding Program, you generally need to use a Medicare-contracted supplier.
Don’t Miss: Does Medicare Become Your Primary Insurance
Home Blood Glucose Monitors
Please Note: This may not be an exhaustive list of all applicable Medicare benefit categories for this item or service.
CIM 60-11
There are several different types of blood glucose monitors that use reflectance meters to determine blood glucose levels. Medicare coverage of these devices varies, with respect to both the type of device and the medical condition of the patient for whom the device is prescribed.
Reflectance colorimeter devices used for measuring blood glucose levels in clinical settings are not covered as durable medical equipment for use in the home because their need for frequent professional re-calibration makes them unsuitable for home use. However, some types of blood glucose monitors which use a reflectance meter specifically designed for home use by diabetic patients may be covered as durable medical equipment, subject to the conditions and limitations described below.
What Are The Key Differences Between The Medicare Version Of Freestyle Libre And G5
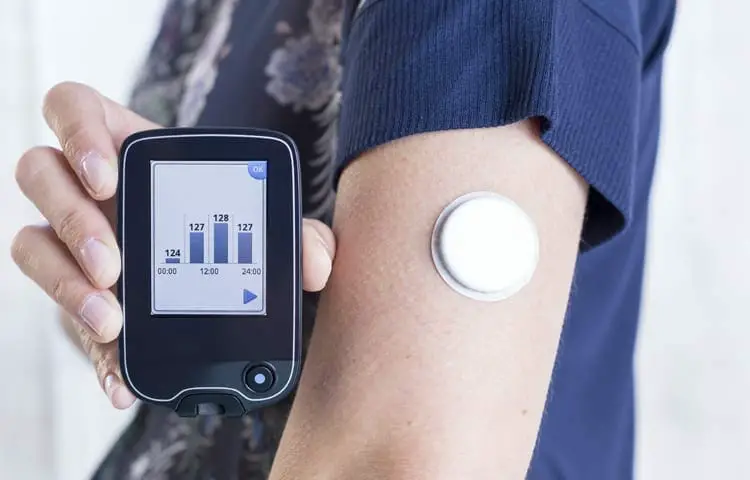
These product differences will change over time some potentially this year. For instance, Dexcoms next-gen G6 is currently under FDA review, with plans to launch before the end of 2018. It is currently under review for one fingerstick calibration per day and 10-day wear, but FDA discussions are also underway for a no-calibration version. Meanwhile, Dexcoms next-next-gen sensor with Verily requires no calibration, will be fully disposable, and last 10 or 14 days. Abbott is currently developing a next-gen FreeStyle Libre as part of its Bigfoot partnership, which will add continuous communication to the FreeStyle Libre sensor. Wed guess it could add alarms too, but this is speculation on our part.
Also Check: What Is The Medicare Part A
Is Dexcom Covered By Medicare
Does Medicare cover Dexcom G6?
Yes. The Dexcom G6 Continuous Glucose Monitoring System is covered by Medicare for patients who meet the Medicare coverage criteria. Medicare coverage for therapeutic CGM includes certain beneficiaries who have either type 1 or type 2 diabetes and intensively manage their insulin. Dexcom now ships Dexcom G6 to Medicare patients with traditional fee-for-service coverage. For a full description of coverage criteria, .
Medicare Coverage Criteria
Medicare patients with type 1 and type 2 diabetes on intensive insulin therapy may be able to obtain reimbursement if the following Medicare coverage criteria are met:
- The patient has diabetes
- The patient is insulin-treated with three or more daily administrations of insulin or a continuous subcutaneous insulin infusion pump
- The patients insulin treatment regimen requires frequent adjustments based on BGM or CGM testing results
- Within six months prior to ordering the CGM, the patient had an in-person visit with the treating practitioner to evaluate their diabetes control and determine that the above criteria have been met and
- Every six months following the initial prescription of the CGM, the patient has an in-person visit with the treating practitioner to assess adherence to their CGM regimen and diabetes treatment plan.
*To view a list of compatible smart devices, visit dexcom.com/compatibility
I am an existing Medicare customer. How do I get my ongoing Dexcom G6 supplies?
CMS Policy
How Much Do Testing Supplies Cost
If youve met your Part B deductible , you will pay 20 percent of the Medicare-approved amount for diabetic testing supplies. Medicare pays the other 80 percent.
Depending on the type of equipment or supplies you need, you may need to:
- rent the equipment.
- buy the equipment.
- choose whether to rent or buy the equipment.
Additionally, your durable medical equipment will only be covered if your doctors and DME suppliers are enrolled in Medicare. You must also purchase your testing supplies from a supplier who accepts assignment. In this case, they can only charge you the coinsurance and Part B deductible.
Accepting assignment means the supplier agrees to be paid directly by Medicare and accepts the payment amount Medicare approves for the service. The Medicare-approved amount is the amount a supplier can be paid by Medicare, and you pay the rest.
To ensure your supplier is enrolled in Medicare, ask if they participate in Medicare before you order the supplies.
How much you will specifically pay for supplies depends on a variety of factors such as:
- Other insurance you may have.
- How much your doctor charges.
- Where you get your supplies.
- Whether your doctor and supplier accept assignment.
Also Check: Does Medicare Cover Nursing Care At Home
Does Medicare Cover Diabetic Sensors
Diabetic sensors are also referred to as glucose sensors. Theyre used to measure blood sugar as part of a CGM system. Medicare does not cover every CGM system. If your system is covered, your diabetic sensor will be, too.
Diabetic sensors are professionally inserted under the skin, usually on the abdomen or arm. They take continual glucose measurements, which you can monitor at a glance. You can share your readings with a mobile device, such as your smartphone.
Are you eligible for cost-saving Medicare subsidies?
Are There Other Options For Medicare Coverage Of Glucose Monitors
Some Medicare beneficiaries choose to receive their Original Medicare benefits through the Medicare Advantage program. Part A covers hospice care when you have a Medicare Advantage plan. Medicare Advantage plans cover glucose monitors in the same way as Medicare Part B and often offer additional benefits, such as vision, dental, and prescription drug coverage. Some types of Medicare Advantage plans use provider networks, and may reduce the amounts you pay to monitor your glucose levels at home with a glucose monitor. In any case, when youre enrolled in a Medicare Advantage plan, youre still in the Medicare program, and must continue paying your Medicare Part B monthly premium, as well as any premium the Medicare Advantage plan may charge.
If you decide to stay with Original Medicare, another option you may have is to sign up for a Medicare Supplement plan to help pay for Original Medicares out-of-pocket costs for glucose monitors and other items and services. Different Medigap plans pay for different amounts of those costs, such as copayments, coinsurance, and deductibles.
You May Like: Does Medicare Cover Home Sleep Apnea Test
What Brand Of Diabetes Supplies Is Covered By Medicare
There are a number of brands of diabetes supplies that are covered by Medicare, specifically:
- OneTouch
- Abbotts
- Bayer
However, not all brands are covered by Medicare, so check your coverage before purchasing any supplies. You can ask your doctor, pharmacist or supplier to check for you, or contact Medicare directly.
Does Medicare Pay For Cgm Accessories And Supplies
Yes, Medicare generally pays 80% of the cost of CGM accessories and supplies. Under the guidelines, Medicare Part B covers:
- Patch refills based on the recommended replacement schedule. For example, if you use a CGM that requires a new patch every 14 days, Medicare will pay for two patches every 28 days.
- Up to 300 test strips every three months if you are being treated with insulin
- Up to 300 lancets every three months if you are being treated with insulin
Medicare Part D may also cover:
- Alcohol swabs
Read Also: How To Get Dental With Medicare
Measurement Of Advanced Glycation End Products By Skin Autoflourescence
Skin autofluorescence is a non-invasive measurement of the level of tissue accumulation of advanced glycation end products , representing cumulative glycemic and oxidative stress. Several studies have shown that AGEs accumulate in skin faster in individuals with poor blood sugar control and that measurement of AGEs by skin autofluorescence may be able to predict the risk of developing diabetes and related complications .
The Scout DS system measures skin AGEs by autoflorescence spectroscopy. The device is a portable desktop system with an arm cradle. The subject places the palm side of their forearm into the cradle and the device shines multiple wavelengths of light into the skin causing the AGEs to fluoresce. The instrument optically calibrates for skin pigmentation, making the measurement impervious to variations in skin color. A specially designed fiber-optic probe sends excitation light to the subject and relays resulting skin fluorescence to the detection module. A value from 0 to 100 representing the likelihood of that subject having an abnormal glucose tolerance test is reported in about 60 seconds. The proposed benefits of the Scout DS system is that the patient would not need to fast or provide a blood sample and results are received much quicker. The system is not intended to replace an oral glucose tolerance test.
What Medicare Members Would Pay
CMS had originally proposed three different categories of payment for those using the different types of CGM technology. The logic was that some didnt require fingersticks so users wouldnt need reimbursement for test strips, as would other systems that still require calibration . It also viewed the FreeStyle Libre flash glucose monitoring a bit differently than other tech, like Dexcom and Eversense. So it proposed different reimbursements for those varying styles of tech.
However, CMS has re-thought that move after public outcry. In its new December 2021 rule comments, the agency noted this:
After consideration of public comments, CMS does not believe it is necessary at this time to further stratify the types of CGMs beyond the two categories of non-adjunctive and adjunctive CGMs.
Also Check: Does Medicare Advantage Cover Chiropractic Care
Who Qualifies For A Continuous Glucose Monitor
Medicare will cover CGMs for people who:
- Have an established diagnosis of Type 1 or Type 2 diabetes
- Are currently using a traditional blood glucose monitor
- Must check their blood sugar a minimum of four times daily
- Use insulin to treat diabetes and require frequent adjustments to their regimens or have a subcutaneous insulin infusion pump
- Receive training from their doctors on how to use CGMs
Diabetes Supplies And Services Covered By Medicare Part B
- Blood glucose testing supplies and equipment
- Insulin pumps and insulin used with a pump
- Diabetes self-management training
- Medical nutrition therapy, including diet and lifestyle counseling
- Hemoglobin A1C tests to monitor blood glucose control
- Foot exams and treatment for diabetes-related nerve damage
- Therapeutic shoes or inserts
Read Also: Does Aarp Medicare Supplement Insurance Cover Hearing Aids
Disposable Blood Glucose Monitors
The ReliOn NewTek has been cleared by the FDA for marketing under the 510 process for persons with diabetes when recommended by their physician. It includes a disposable meter containing 100 test strips plus control solution. The ReliOn NewTek received FDA 510 marketing clearance in 2003. According to the FDA 510 summary letter submitted by the manufacturer to the FDA, testing demonstrated that its performance was substantially equivalent to the Hypoguard Advance Blood Glucose Monitoring System.
What Brands Are Covered
Most brands currently on the market are covered through Medicare. This includes Medtronic, Dexcom, Eversense and Freestyle Libre 1 and 2. In the past, Medicare only covered non-adjunctive monitors, which dont require a finger stick glucose check to confirm findings. The new rules allow beneficiaries to also get adjunctive monitors, where users perform a finger stick test to ensure accuracy before making dosing decisions.
Read Also: Does Medicare Part D Cover Shingrix Vaccine
How Much Does It Cost To Get An Insulin Pump
Medicare covers 80% of the Medicare-approved cost of insulin pumps. You are responsible for the other 20%, plus the Part B deductible and monthly premiums.
Your pump must be prescribed by a Medicare-approved physician and purchased or rented from a Medicare-approved medical supplier for Medicare to cover it.
Does Medicare Cover Dexcom G6
Glucose monitoring technology, including continuous glucose monitoring systems, plays a vital role in protecting the health of individuals who suffer from issues related to blood sugar and insulin production. In the past, individuals who needed to check their blood glucose levels would typically need to obtain a blood sample, often through a prick on the finger, and the sample would then need to be manually inserted into a testing device for analysis. Unfortunately, this method can be time-consuming, and it relies on the user remembering to take measurements at various times throughout the day. This could also mean missing regular testing during the night due to sleep.
Today, however, advanced glucose monitoring using technology like the Dexcom G6 can provide real-time data 24 hours a day for people who need continuous monitoring. Modern monitoring provides important information regarding overall health as it relates to glucose levels, allowing for faster treatment in the event of an emergency. This is vital for patients who are diabetic or for patients who may be at risk for developing health conditions related to fluctuating or uneven blood glucose.
Related articles:
Also Check: Which Is Better Medicare Supplement Or Medicare Advantage
Glutamic Acid Decarboxylase Autoantibodies
Aetna considers measurement of autoantibodies to GAD medically necessary for distinguishing type 1 from type 2 diabetes when the clinical history is ambiguous and the results of testing will influence patient management. Measurement of anti-GAD antibodies is also considered medically necessary in diagnosing stiff-person syndrome. Anti-GAD antibody measurement is considered experimental and investigational for predicting the onset of diabetes and for all other indications.
Does Medicare Cover Cpap Masks And Sleep Apnea Supplies
While obstructive sleep apnea and insulin resistance may be linked, not all people with diabetes on Medicare are eligible for CPAPs and related accessories. First, youll need to be diagnosed with obstructive sleep apnea in that case, Medicare may cover a three-month CPAP trial. If your doctor finds that this therapy improves your condition, Medicare could also cover CPAP supplies in the long term.
Are you living with diabetes and sleep apnea? If so, explore US MEDs selection of CPAP accessories. We sell nasal pillow masks, nasal masks, and full-face masks for CPAP users.
If youre still wondering about Medicare coverage for US MED products, wed be happy to help! Give us a call at 1-877-840-8218 to discuss this topic or any other questions you may have.
Recommended Reading: Can I Keep Medicare If I Get A Job
Continuous Glucose Monitoring Devices
Aetna considers the short-term diagnostic use of continuous glucose monitoring devices medically necessary for persons with diabetes who have either of the following problems in controlling blood glucose level, unresponsive to conventional insulin dose adjustment:
Aetna considers the short-term diagnostic use of continuous glucose monitoring devices medically necessary to diagnose primary islet cell hypertrophy or persistent hyperinsulinemic hypoglycemia of infancy in persons with symptoms suggestive of recurrent hypoglycemia.For short-term diagnostic use, no more than 2 continuous glucose monitoring periods are considered medically necessary within a 12-month period.
Aetna considers experimental and investigational the long-term use of continuous glucose monitors for individuals with type 1a glycogen storage disease, persons with type 2 diabetes not using intensive insulin regimens, nesidioblastosis , neonatal hypoglycemia, and for monitoring blood glucose in non-diabetic persons following gastric bypass surgery because there is insufficient evidence of the clinical benefits of this approach for these indications.
Therapeutic Continuous Glucose Monitors
Medicare covers therapeutic continuous glucose monitors and related supplies instead of blood sugar monitors for making diabetes treatment decisions, like changes in diet and insulin dosage.
If you use insulin and require frequent adjustments to your insulin regimen/dosage, Medicare may cover a continuous glucose monitor if your doctor determines that you meet all of the requirements for Medicare coverage.
Recommended Reading: Is Keystone 65 A Medicare Advantage Plan
Why Medicare Will Cover The Medtronic Cgm Now
Up until now, Medtronic has been the only CGM company without Medicare coverage.
The other CGM products available Dexcom G5 and G6, Abbott FreeStyle Libre 2, and the implantable Eversense CGM from Senseonics and Ascensia have been covered for years. But Medtronic is the only company that did not get a non-adjunctive designation , which wouldve allowed the CGM to be used for insulin dosing and treatment decisions without a need for confirmatory fingersticks.
That so-called dosing claim was a new category created by the Food and Drug Administration , its first attempt to distinguish the different levels of CGM technology that existed at that time in 2017. The Dexcom G5 was the first to obtain that status and be known as a therapeutic CGM, followed by the Abbott FreeStyle Libre and then Eversense 90-day implantable CGM.
To date, Medtronics Guardian CGM remains the only one that requires fingerstick calibrations and doesnt have Medicare coverage.
But CMS is now changing that, lumping Medtronics device into the same category as the non-adjunctive devices so that they are all covered by Medicare.
Importantly, the new Medicare policy does not include Medtronics stand-alone Guardian Connect CGM system. Instead, it only allows for Medicare coverage of the Medtronic CGM when it is combined with the companys MiniMed insulin pumps.
