Watchman Implant Reduces Risk Of Stroke In Afib Patients
At Orlando Health, our doctors are trained in the newest surgical techniques and treatments to offer our patients more options for improved health and quality of life. Patients with atrial fibrillation who cannot be treated with the blood thinner warfarin due to bleeding concerns can now receive a minimally invasive procedure called the Watchman implant to reduce their risk of stroke.
Watchman is a one-time procedure approved by the Food and Drug Administration that reduces the risk of stroke in people with non-valvular atrial fibrillation . According to clinical studies, the Watchman implant is as effective at reducing the risk of stroke as warfarin . But unlike warfarin, the procedure also reduces the long-term risk of bleeding. Newer blood thinners offer an option to warfarin, but they dont take away the long-term risk of bleeding. The Watchman procedure may be an option for patients who:
- Have AFib not caused by heart valve problems
- Have been recommended to take blood thinning medicines by their doctor
- Can take warfarin but need an alternative to blood thinners because they have a history of bleeding or a lifestyle that puts them at risk for bleeding from long term therapy
But Who To Include And How To Follow Them Are Still Fluid Issues
There are three key issues that will impact use of Watchman and future left atrial appendage closure devices for prevention of stroke in atrial fibrillation patients — who is covered, how patients will be followed, and with what comparator.
Who Is Covered
The Centers for Medicare and Medicaid Services issued a draft National Coverage Determination that Watchman is not reasonable or necessary, so it is not covered by Medicare. But CMS is proposing to cover Watchman under a Coverage with Evidence Development paradigm. However, CMS created some confusion about just which atrial fibrillation patients would be eligible for CED coverage of this device.
The FDA approval specified patients had to be able to take warfarin, and the NCD said it would be covered to patients for whom warfarin is contraindicated. CMS has since clarified that the FDA label is broader than the proposed Medicare coverage determination, but that Medicare proposes to cover what the FDA approved and not any off-label use.
Thus, it is likely that the confusion over eligible patients will be resolved or clarified in the final NCD. It appears that CMS simply wants to ensure that the device is “necessary,” not just a convenient replacement for the need to take daily pills.
Brindis — who is the senior medical officer, external affairs, for the ACC-National Cardiovascular Data Registry — said there are still a lot of unanswered questions about LAA closure that need answering:
How Patients Will Be Followed
How The Procedure Works
The Watchman implant is designed to close the left atrial appendage in the heart to keep harmful blood clots from entering the blood stream and potentially causing a stroke. In this minimally invasive procedure, the device is implanted like a stent, by means of a narrow tube inserted through a small incision in the upper leg. The surgeon guides the implant through the tube and places it in the left chamber of the heart to close the left atrial appendage.
The procedure is done under general anesthesia and takes about an hour. Patients commonly stay in the hospital overnight and leave the next day. The Watchman device is a one-time implant and does not need to be replaced.
You May Like: Is Humana Medicare Part D
Finding A Watchman Medical Center
More than 650 of the nations top medical centers perform the WATCHMAN Implant. If you and your cardiologist decide that the WATCHMAN Implant is right for you, you may be referred to one of these centers. At the medical center you and your cardiologist select, a team of trained specialists will place the WATCHMAN Implant in your heart. About 45 days after your procedure, one of the specialists will check your heart to see whether you can stop taking blood thinners. Its important to understand that neither the WATCHMAN Implant nor blood thinners cure atrial fibrillation or its symptoms, like irregular heart rhythm. After getting the WATCHMAN Implant, youll continue to see your cardiologist to manage your AFib.
What Is The Success Rate Of The Watchman Procedure
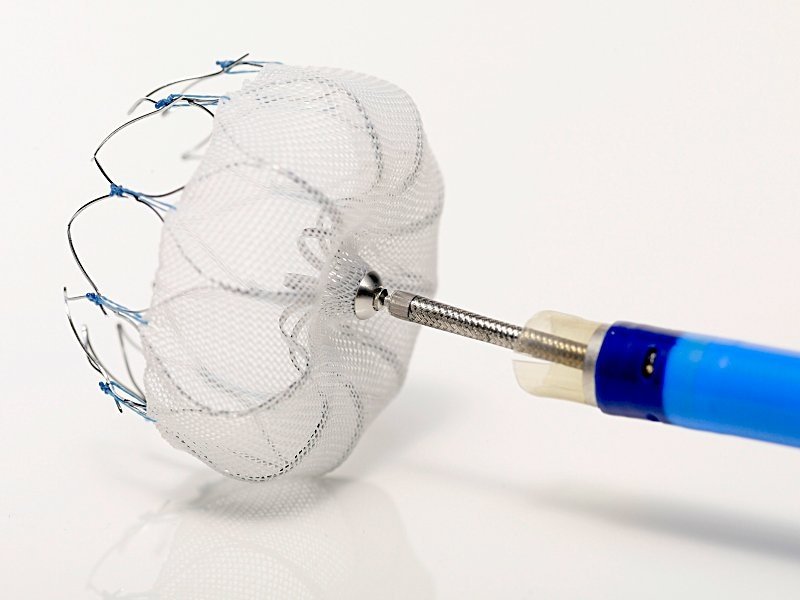
Several research studies have been performed to investigate the efficacy of the WATCHMAN procedure. In a 5-year study conducted to compare the WATCHMAN procedure to traditional treatment with warfarin, it was found that the procedure was as effective as anticoagulant treatment. Additionally, patients who had the WATCHMAN procedure performed were less likely to experience significant bleeds and hemorrhages.
In a study done in 2020, researchers explored the complication rate in patients who underwent a WATCHMAN procedure within a two-year period. In the study, 38,158 WATCHMAN procedures were completed across America. In these cases, 98.1% of cases were implanted correctly, and the in-hospital complication rate was 2.16%. Of these complications, the most common complications were pericardial effusion requiring intervention and major bleeding , whereas stroke and death were rare.
Additionally, another study done in 2021 assessed the safety of the newer version of the device, the WATCHMAN FLX LAA closure device. Of the 400 patients in the study, 100% experienced primary effectiveness from the procedure, and only seven patients experienced minor complications afterward. Of these 7 complications, device-related thrombus was reported in 7 patients, no patients experienced pericardial effusion requiring open cardiac surgery, and there were no device embolizations. This study demonstrates the overall improved safety of the latest generation, WATCHMAN FLX device.
You May Like: What Is A Ppo Medicare Plan
How Much Does The Watchman Cost
The cost of the WATCHMAN implant greatly depends on your insurance and medical coverage. Please speak with your medical professionals and medical insurance provider to learn more about the out-of-pocket costs of undergoing a WATCHMAN procedure.
A 2018 study looked at the cost effectiveness of a WATCHMAN implant procedure over a period of 10 years, when compared to 10 years of being on a blood thinner. Upfront procedure costs initially make the WATCHMAN procedure more expensive than warfarin and other blood thinners. However, over time, within 10 years, the WATCHMAN procedure delivers more quality-adjusted life years and has lower total costs when compared to the long-term costs of blood thinning medications.
Why Dont All Patients With Atrial Fibrillation Have The Watchman Device Implanted
The recommendation for the use of the Watchman device is based on evidence gathered over many years and in thousands and thousands of patients. As things stand, the evidence is there only for patients that dont tolerate blood thinner. There is no good evidence currently that atrial fibrillation who are doing well taking blood thinning medication should have the Watchman Device implanted.
Also Check: Does Medicare Cover Autologous Serum Eye Drops
What Is The Watchman Procedure
Watchman is a device that is about the size of a quarter. The device is implanted into the LAA of the heart in people with non-valvular Afib in order to keep blood clots from escaping and causing a stroke.
The procedure is done by a cardiologist, who makes a small incision in the upper leg, inserts a narrow tube, and guides the implant into the LAA. The Watchman is a minimally invasive, permanent, one-time procedure that lasts a lifetime.
The Watchman procedure is done under general anesthesia and usually requires an overnight hospital stay. While the procedure is done so that people can stop taking warfarin , the drug will need to continue to be taken for about 45 days after the surgery, or until the LAA is permanently closed off. During that time, heart tissue will grow over the implant to provide a barrier against blood clots.
Estimated Medicare Patient Out
Note: Estimates are based on 2022 Medicare Fee for Service rates. These estimates will vary depending upon the patients individual healthcare policy. Insurance coverage can vary significantly from one plan to another, even within the same insurance company. We therefore recommend that patients contact their insurance provider directly with questions regarding estimated patient-specific out-of-pocket costs.
Health economic and reimbursement information provided by Boston Scientific Corporation is gathered from third-party sources and is subject to change without notice as a result of complex and frequently changing laws, regulations, rules, and policies. This information is presented for illustrative purposes only and does not constitute reimbursement or legal advice. Boston Scientific encourages providers to submit accurate and appropriate claims for services.
Boston Scientific does not promote the use of its products outside their FDA-approved label. Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements.
References:
Also Check: Does Aarp Offer Medicare Supplement Insurance
The Watchman Procedure Does Appendage Type Matter
The Watchman Device is designed to cover and essentially eliminate the appendage, basically preventing clots from forming there and reducing stroke risk. The video below shows the three main appendage types. Chicken-wing, broccoli and windsock types. Yes these names appear ridiculous when describing anatomy however they accurately describe the shape of the appendage. The easiest to do is the windsock type, as this is easier to deliver. The chicken wing can sometimes be straightforward and sometimes more difficult. The broccoli is felt to be the most difficult. Regardless successful use of the Watchman Device has been described in all these circumstances.
Where Cms Misses The Mark
CMS has proposed that patients meet three requirements in order to qualify for reimbursement for WATCHMAN: a high CHADS2 or CHA2DS2-VASc score, a high HAS-BLED score and a contraindication to warfarin. Dr. Wazni notes this will eliminate an important segment of the population who could benefit from the device.
Take a patient with a high CHA2DS2-VASc score who had an intracranial bleed on warfarin, he says. This patient has a long-term contraindication to warfarin but a low HAS-BLED score of 1. Wed like to see CHA2DS2-VASc score as the primary driving factor, and either a high HAS-BLED score or a contraindication to anticoagulants as the second condition.
Don’t Miss: When Do You Stop Paying Medicare And Social Security Taxes
What Is The Typical Recovery Time From A Watchman Procedure
Because this procedure is minimally invasive, the recovery time for this procedure is often relatively short. Patients are usually kept overnight in the hospital for observation, then able to return home the next day. Patients can return to regular activities within a couple of weeks after the procedure.
Who Should Not Get A Watchman Device

Even in those patients who have already fulfilled the above criteria, the Watchman Device should not be used in the following groups of patients.
Intracardiac thrombus is visualized by echocardiographic imaging. This means that patients who have been found to have clots in the heart should not have the device. This is because the Watchman Procedure itself would be associated with a high risk of the clot dislodging and a stroke or other major complication.
An atrial septal defect repair or closure device or a patent foramen ovale repair or closure device is present. In patients that have had a history of a hole in the heart and a device already used to close it, the Watchman Procedure is not typically a good idea as the existing device will simply get in the way of the procedure.
The LAA anatomy will not accommodate a device. Remember the Watchman Device is designed for placement in the left atrial appendage. Although in most cases the size and shape of the appendage is ok, in some cases it is not able to accommodate the device. This should be known prior to the procedure.
Miscellaneous reasons. Reasons may include inability to use a TEE probe for imaging, the presence of active bleeding or infection, anatomy that does not allow catheters to be used appropriately, the patient has a hypersensitivity to the components of the device, etc. Patients should typically be able to use aspirin or Plavix.
Read Also: Are Motorized Wheelchairs Covered By Medicare
Us Medicare To Cover Boston Scientific Watchman Left Atrial Appendage Closure Device
The US Centers for Medicare and Medicaid Services are to cover percutaneous left atrial appendage closure therapy under specific criteria, as outlined in the agencys final National Coverage Determination . This decision, effective immediately, provides consistent and uniform access to the Watchman LAAC Device as a non-pharmacological treatment option for stroke risk reduction for appropriate Medicare beneficiaries.
We are very pleased CMS has established national coverage for this life-changing therapy for Medicare beneficiaries who have a reason to seek an alternative to long-term anticoagulation, says Mike Mahoney, president and chief executive officer, Boston Scientific.
The Watchman device, the first and only percutaneous LAAC therapy approved by the US Food and Drug Administration , is indicated for patients with non-valvular atrial fibrillation who are at high stroke risk, suitable for warfarin, and are seeking an alternative to long-term warfarin therapy.
According to the NCD, CMS will cover percutaneous LAAC therapy when specific conditions are met. CMS adopted the majority of physician and professional medical society feedback received in the 30-day public comment period, specifically as it relates to patient coverage criteria and future data collection requirements.
What Are The Proposed Advantages Of The Watchman Device Implant
Patients with the Watchman Device can typically stop strong blood thinning medication such as Coumadin 45 days after the device is implanted therefore decreasing the chance of bleeding complications and the need to take daily medicines. There is the reduced cost associated with not needing to take a lifetime worth of blood thinner.
Recommended Reading: Does Medicare Cover While Traveling Abroad
Primary And Secondary Outcomes
The primary outcome was mortality during admission and within 1 year of LAAC. The secondary outcomes were readmissions within 30 days after LAAC discharge and reasons for readmission among patients discharged alive. To assess reasons for readmission, all first readmissions within 30 days of discharge were categorized according to the primary diagnosis code using the US Agency for Healthcare Research and Quality Clinical Classification System,9 which collapses individual ICD-9-CM and ICD-10 codes into approximately 200 meaningful clinical categories. In addition, we evaluated the likelihood of admission for ischemic stroke or TIA within 180 days and the rate of admission for ischemic stroke or TIA per patient-year among patients discharged alive for whom at least 6 months of follow-up data were available. Admissions for ischemic stroke were identified by primary diagnosis on inpatient claims using ICD-10 codes starting with I63, I65, and I66 for ischemic stroke and G45 for TIA.
What Does The Watchman Device Do
Looking similar to a small parachute or umbrella, the WATCHMAN device is a medical implant, meaning that it is left in the body after the procedure is completed. The Watchman device is inserted into the left atrial appendage of the heart.
In the left atrium, there is a small sac called the left atrial appendage . When the heart is functioning normally, the muscle squeezes hard enough to remove all blood from the sac, and no issues arise. But, in patients with AFib, the irregular heart rhythm can cause blood to collect in the LAA, which may cause the formation of a blood clot. If dislodged, this clot can leave the heart and cause a stroke.
The WATCHMAN device acts as a blockade to the left atrial appendage once it is placed into the left atrium. Inserted through a minimally invasive catheter , the WATCHMAN device is guided up into the heart and inserted directly into the left atrial appendage. Once implanted, any clotted blood left in the LAA cannot escape, reducing the risk of developing a stroke.
A WATCHMAN procedure is usually performed under anesthesia the procedure typically takes less than an hour to do. Patients typically stay in the hospital one night and are able to be discharged to home the following day.
Long-term, patients are able to stop taking strong blood thinning medications, while maintaining the benefits of stroke risk reduction, and reducing the risks of major bleeding.
You May Like: Does Medicare Cover Eye Surgery
Ama Disclaimer Of Warranties And Liabilities
CPT is provided as is without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. The AMA warrants that due to the nature of CPT, it does not manipulate or process dates, therefore there is no Year 2000 issue with CPT. The AMA disclaims responsibility for any errors in CPT that may arise as a result of CPT being used in conjunction with any software and/or hardware system that is not Year 2000 compliant. No fee schedules, basic unit, relative values or related listings are included in CPT. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this file/product is with Palmetto GBA or CMS and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
CMS Disclaimer
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled I Accept.
Watchman Medicare Coverage Missing Patient Decisionmaking Tool
The CMS’ final coverage rule on Boston Scientific’s cardiac device the Watchman does not include language requiring patients and physicians consult on the benefits and risks of the device, disappointing advocates who were hoping to empower patients.
In its proposed coverage decision last year, the CMS stated the patient and provider must use an evidence-based decision tool to determine the appropriateness of anticoagulation therapy. It urged conversation and consultation regarding the benefits and harms of Watchman.
However, when the CMS finalized its decision for Medicare coverage for the device this month, the requirement that the patient and doctor discuss the benefits and risks of device had been removed.
Michael Weinstein, a JPMorgan Analyst who specializes in cardiovascular medical technology, noted the change in a Feb. 9 investor’s note as a less restrictive requirement on shared decisionmaking.
Informed Medical Decisions Foundation, a Boston-based organization that advocates for evidence-based shared decisionmaking between doctors and patients, found the final wording vague and said it hoped future coverage decision on products would more clearly stress shared decisionmaking.
Others said they were not so sure that the section had been weakened, and in fact felt that it may have been strengthened.
The aids include a description of the benefits and harms of a product or procedure.
Great Britain and Canada are far ahead of the U.S. in the use of aids.
Recommended Reading: What Is A Medicare Point Of Service Plan
